Coronavirus
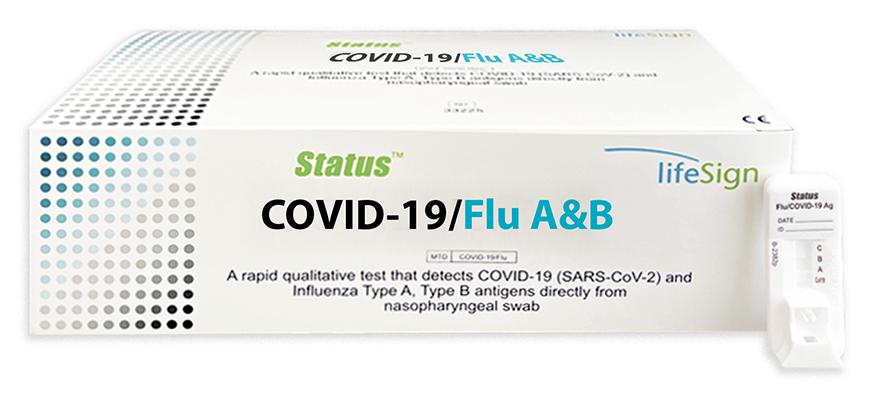
Status COVID-19/Flu A&B
Product Highlights
- Sensitivity and Specificity: ? COVID-19 - Sensitivity 93.9%, Specificity 100%rn? Flu A - Sensitivity 91.4%, Specificity 95.7%rn? Flu B - Sensitivity 87.6%, Specificity 95.9%
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
Manufacturer: LifeSign
Item#: GRM33225
Status COVID-19/Flu A&B
Packaging:
25/BX
Unit of Measure:
Price:

Product Description
A Rapid Immunoassay for the Simultaneous Direct Detection and Differential Diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from nasopharyngeal swab specimens.
» Sensitivity and Specificity: ? COVID-19 - Sensitivity 93.9%, Specificity 100%rn? Flu A - Sensitivity 91.4%, Specificity 95.7%rn? Flu B - Sensitivity 87.6%, Specificity 95.9%
» FDA Emergency Use Authorization (EUA)
» Visually read in 15 minutes
» Flocked nasopharyngeal swab for superior specimen collection and patient comfort
Additional Information
| Item Number | GRM33225 |
| Packaging | 25/BX |
Recently Viewed Items